The Role of Aspirin and Its Derivatives in Enhancing Broiler Health and Performance: A Comprehensive Review
Keywords:
Aspirin, Acetylsalicylic acid (ASA), Diet, Performance, Broiler, SupplementAbstract
Following restrictions on antibiotic growth promoters (AGPs) in many regions due to their link to the development of microbial resistance. The recent ban on antibiotics in poultry has underscored the urgent need for alternative methods to enhance growth performance in chickens. Various innovative strategies are being explored to address this challenge. Aspirin, chemically known as acetylsalicylic acid, has attracted attention in poultry nutrition, particularly in broiler diets. Its potential benefits include enhancing growth performance, improving feed efficiency, and possibly providing anti-inflammatory effects. This review synthesizes findings from 64 studies conducted between 2015 and 2025 on the effects of aspirin use across many animal species, particularly in broiler diets, focusing on growth and development, carcass traits, and health criteria. The literature indicates that the addition of Aspirin to drinking water at concentrations exceeding 200 mg/L was associated with reduced performance and adverse histology in several studies. Conversely, including up to 100 mg/kg of Aspirin in the diet has been demonstrated in several studies to improve performance and decrease the population of Escherichia coli. These effects are especially pronounced under stressful conditions commonly faced in broiler production, such as high stocking densities and heat stress. This review underscores the health advantages and potential uses of Aspirin and its derivatives in broiler nutrition.
Downloads
References
Capita R, Alonso-Calleja C. Antibiotic-resistant bacteria: a challenge for the food industry. Critical reviews in food science and nutrition. 2013;53(1):11-48. [PMID: 23035919] [DOI]
Vidovic N, Vidovic S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics. 2020;9(2):52. [PMID: 32023977] [PMCID: PMC7168261] [DOI]
Zamojska D, Nowak A, Nowak I, Macierzyńska-Piotrowska E. Probiotics and postbiotics as substitutes of antibiotics in farm animals: A review. Animals. 2021;11(12):3431. [PMID: 34944208] [PMCID: PMC8697875] [DOI]
Bueno I, Ricke I, Hwang H, Smith E, Nault A, Johnson TJ, et al. Efficacy of antibiotic and non-antibiotic interventions in preventing and treating necrotic enteritis in broiler chickens: A Systematic Review. Avian diseases. 2023;67(1):20-32. [PMID: 37140108] [DOI]
Kaur B, Singh P. Inflammation: Biochemistry, cellular targets, anti-inflammatory agents and challenges with special emphasis on cyclooxygenase-2. Bioorganic Chemistry. 2022;121:105663. [PMID: 35180488] [DOI]
Rossoni G, Manfredi B, Colonna VDG, Bernareggi M, Berti F. The Nitroderivative of Aspirin, NCX 4016, Reduces Infarct Size Caused by Myocardial Ischemia-Reperfusion in the Anesthetized Rat. The Journal of Pharmacology and Experimental Therapeutics. 2001;297(1):380-7. [PMID: 11259566] [DOI]
Jung KJ, Kim JY, Zou Y, Kim YJ, Yu BP, Chung HY. Effect of short-term, low dose aspirin supplementation on the activation of pro-inflammatory NF-κB in aged rats. Mechanisms of Ageing and Development. 2006;127(3):223-30. [PMID: 16310244] [DOI]
Mondal S, Jana M, Dasarathi S, Roy A, Pahan K. Aspirin ameliorates experimental autoimmune encephalomyelitis through interleukin-11–mediated protection of regulatory T cells. Science Signaling. 2018;11(558):eaar8278. [PMID: 30482850] [PMCID: PMC6325078] [DOI]
Zhao R, Coker OO, Wu J, Zhou Y, Zhao L, Nakatsu G, et al. Aspirin Reduces Colorectal Tumor Development in Mice and Gut Microbes Reduce its Bioavailability and Chemopreventive Effects. Gastroenterology. 2020;159(3):969-83.e4. [PMID: 32387495] [DOI]
Bulckaen H, Prévost G, Boulanger E, Robitaille G, Roquet V, Gaxatte C, et al. Low-dose aspirin prevents age-related endothelial dysfunction in a mouse model of physiological aging. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294(4):H1562-H70. [PMID: 18223195] [DOI]
Guo Y, Wang Q-z, Tang B-s, Zuo Y-f, Li F-m, Jiang X, et al. Effects of aspirin on atherosclerosis and the cyclooxygenase-2 expression in atherosclerotic rabbits. Chinese Medical Journal. 2006;119(21). [DOI]
Batista RP, Denadai R, Saad-Hossne R. Effects of aspirin on mesenteric lymph nodes of rabbits as basis for its use on lymph nodes metastases. Acta Cirúrgica Brasileira. 2012;27:795-801. [PMID: 23117612] [DOI]
Herbert J-M, Dol F, Bernat A, Falotico R, Lalé A, Savi P. The antiaggregating and antithrombotic activity of clopidogrel is potentiated by aspirin in several experimental models in the rabbit. Thrombosis and haemostasis. 1998;80(09):512-8. [PMID: 9759636] [DOI]
Cook JC, Jacobson CF, Gao F, Tassinari MS, Hurtt ME, DeSesso JM. Analysis of the nonsteroidal anti-inflammatory drug literature for potential developmental toxicity in rats and rabbits. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2003;68(1):5-26. [PMID: 12852480] [DOI]
Dong Z, Yang L, Jiao J, Jiang Y, Li H, Yin G, et al. Aspirin in combination with gastrodin protects cardiac function and mitigates gastric mucosal injury in response to myocardial ischemia/reperfusion. Frontiers in Pharmacology. 2022;13:995102. [PMID: 36238560] [PMCID: PMC9553090] [DOI]
Zhang M, Xia F, Xia S, Zhou W, Zhang Y, Han X, et al. NSAID-associated small intestinal injury: an overview from animal model development to pathogenesis, treatment, and prevention. Frontiers in Pharmacology. 2022;13:818877. [PMID: 35222032] [PMCID: PMC8864225] [DOI]
Takahashi O, Hiraga K. Differences in the Haemorrhagic Toxicity of Aspirin between Rats and Mice. Acta Pharmacologica et Toxicologica. 1985;56(1):6-13. [PMID: 3872009] [DOI]
Al-Abdaly Y, Saeed M, Al-Hashemi H. Effect of methotrexate and aspirin interaction and its relationship to oxidative stress in rats. 2021. [DOI]
Du H, Xu F, Liu J, Zhang J, Qin Y, Xu Y, et al. Long-term aspirin administration suppresses inflammation in diabetic cystopathy. Aging. 2023;15(17):9128-43. [PMID: 37702622] [PMCID: PMC10522387] [DOI]
Pozniak B, Switala M, Jaworski K, Okoniewski P, editors. Comparative pharmacokinetics of acetylsalicylic acid and sodium salicylate in chickens and turkeys. JOURNAL OF VETERINARY PHARMACOLOGY AND THERAPEUTICS; 2012: WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030-5774, NJ USA.
Protasiuk E, Olejnik M. Residues of salicylic acid and its metabolites in hen plasma, tissues and eggs as a result of animal treatment and consumption of naturally occurring salicylates. Food Additives & Contaminants: Part A. 2020;37(6):946-54. [PMID: 32240053] [DOI]
Ferronato G, Tavakoli M, Bouyeh M, Seidavi A, Suárez Ramírez L, Prandini A. Effects of combinations of dietary vitamin C and acetylsalicylic acid on growth performance, carcass traits and, serum and immune response parameters in broilers. Animals. 2024;14(4):649. [PMID: 38396617] [PMCID: PMC10886125] [DOI]
Tavakoli M, Bouyeh M, Seidavi A. Influences of dietary aspirin supplementation on growth performance, carcass characteristics and gastrointestinal organs of broilers. Journal of the Hellenic Veterinary Medical Society. 2022;73(4):5061-6. [DOI]
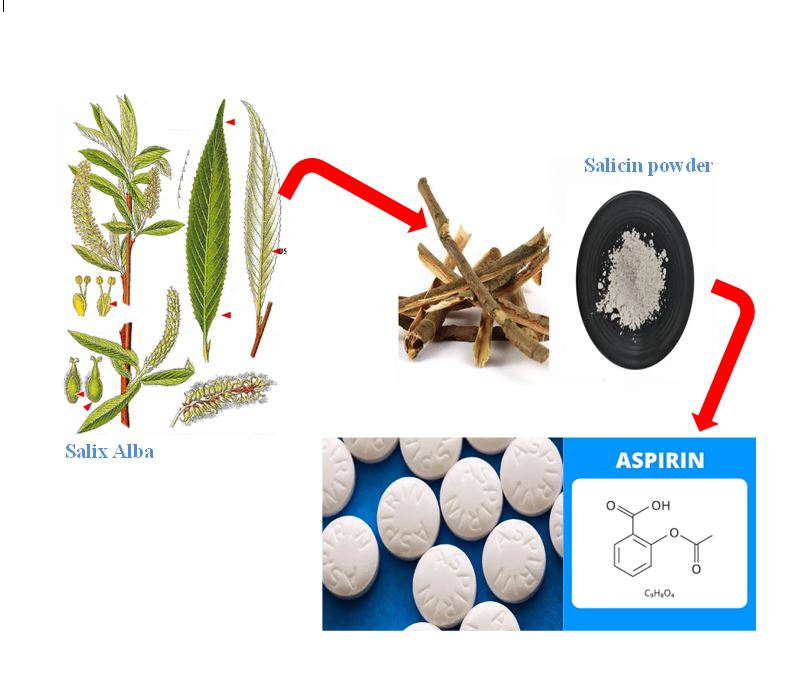
Panaite TD, Saracila M, Papuc CP, Predescu CN, Soica C. Influence of Dietary Supplementation of Salix alba Bark on Performance, Oxidative Stress Parameters in Liver and Gut Microflora of Broilers. Animals. 2020;10(6):958. [PMID: 32486449] [PMCID: PMC7341264] [DOI]
Saracila M, Panaite TD, Predescu NC, Untea AE, Vlaicu PA. Effect of dietary salicin standardized extract from salix alba bark on oxidative stress biomarkers and intestinal microflora of broiler chickens exposed to heat stress. Agriculture. 2023;13(3):698. [DOI]
Fathi M, Haydari M, Tanha T. Influence of dietary aspirin on growth performance, antioxidant status, and mortality due to ascites in broiler chickens. 2016.
Almeida E, Górniak S, Di Gregorio M, Araújo C, Andréo-Filho N, Momo C, et al. Safety and growth-promoting potential of repeated administration of sodium salicylate to broilers. Animal-Open Space. 2022;1(1):100026. [DOI]
Poźniak B, Świtała M, Bobrek K, Graczyk S, Dzimira S. Adverse effects associated with high-dose acetylsalicylic acid and sodium salicylate treatment in broilers. British poultry science. 2012;53(6):777-83. [PMID: 23398422] [DOI]
Saker OA, El-Dakroury MF, Al-Sokary ET, Risk WF, Abass ME. Ameliorative Effect of Dietary Acetylsalicylic Acid and Sodium Bicarbonate Supplementation on Growth and Health Status of broiler chicks exposed to heat-stress. Alexandria Journal of Veterinary Sciences. 2020;64(1). [DOI]
Dabai S, Bello S, Dabai J. Growth performance and carcass characteristics of finisher broiler chickens served carrot leaf extract as a supplementary source of vitamins and minerals. Nigerian Journal of Animal Science. 2021;23(1):144-9.
Panaite T, Saracila M, Papuc C, Predescu C, Soica C. Influence of dietary supplementation of Salix alba bark on performance, oxidative stress parameters in liver and gut microflora of broilers. Animals 2020; 10: 958. 2020. [PMID: 32486449] [PMCID: PMC7341264] [DOI]
Di Gregorio MC, de Almeida ERM, Momo C, da Silva Araújo CS, Hueza IM, Andréo-Filho N, et al. Sodium Salicylate as Feed Additive in Broilers: Absence of Toxicopathological Findings. Animals. 2023;13(9):1430. [PMID: 37174467] [PMCID: PMC10177601] [DOI]
Zhong J, Zhen W, Bai D, Hu X, Zhang H, Zhang R, et al. Effects of aspirin eugenol Ester on liver oxidative damage and energy metabolism in immune-stressed broilers. Antioxidants. 2024;13(3):341. [PMID: 38539874] [PMCID: PMC10967847] [DOI]
AL-Tamimy S. The Effect of Adding Tartaric Acid and Salicylic Acid on the Chemical Composition of Eggs Produced from Lohman Chickens. Archives of Razi Institute. 2022;77(6):2353.
Muneeb M, Javed MT, Sarfaraz T, Ayub A, Israr F, Anwar S, et al. Effects of different acetylsalicylic acid doses on body organs, histopathology, and serum biochemical parameters in broiler birds. Brazilian Journal of Veterinary Research and Animal Science. 2022;59:e176998-e. [DOI]
Zhang H, Zhang Y, Bai D, Zhong J, Hu X, Zhang R, et al. Effect of dietary aspirin eugenol ester on the growth performance, antioxidant capacity, intestinal inflammation, and cecal microbiota of broilers under high stocking density. Poultry Science. 2024;103(7):103825. [PMID: 38772090] [PMCID: PMC11131080] [DOI]
Tawfeek N, Mahmoud MF, Hamdan DI, Sobeh M, Farrag N, Wink M, et al. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Frontiers in pharmacology. 2021;12:593856. [PMID: 33643045] [PMCID: PMC7908037] [DOI]
Liu X, Tao Q, Shen Y, Liu X, Yang Y, Ma N, et al. Aspirin eugenol ester ameliorates LPS-induced inflammatory responses in RAW264. 7 cells and mice. Frontiers in Pharmacology. 2023;14:1220780. [PMID: 37705535] [PMCID: PMC10495573] [DOI]
Huang MZ, Yang YJ, Liu XW, Qin Z, Li JY. Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. British Journal of Pharmacology. 2019;176(7):906-18. [PMID: 31583045] [PMCID: PMC6754946] [DOI]
Huang M-Z, Yang Y-J, Liu X-W, Qin Z, Li J-Y. Aspirin Eugenol Ester Reduces H2O2‐Induced Oxidative Stress of HUVECs via Mitochondria‐Lysosome Axis. Oxidative Medicine and Cellular Longevity. 2019;2019(1):8098135. [PMID: 30706438] [PMCID: PMC6433644] [DOI]
Saitoh H, Sakaguchi M, Miruno F, Muramatsu N, Ito N, Tadokoro K, et al. Histopathological Analysis of Lipopolysaccharide-Induced Liver Inflammation and Thrombus Formation in Mice: The Protective Effects of Aspirin. Current Issues in Molecular Biology. 2024;46(12):14291-303. [PMID: 39727984] [PMCID: PMC11674652] [DOI]
Zimmermann P, Curtis N. Antimicrobial effects of antipyretics. Antimicrobial Agents and Chemotherapy. 2017;61(4):10.1128/aac. 02268-16. [PMID: 28137805] [PMCID: PMC5365702] [DOI]
Wang WH, HU FL, CY WONG B, Berg DE, LAM SK. Inhibitory effects of aspirin and indometacin on the growth of Helicobacter pylori in vitro. Chinese Journal of Digestive Diseases. 2002;3(4):172-7. [DOI]
Ríos-Ibarra CP, Lozano-Sepulveda S, Muñoz-Espinosa L, Rincón-Sánchez AR, Cordova-Fletes C, Rivas-Estilla AMG. Downregulation of inducible nitric oxide synthase (iNOS) expression is implicated in the antiviral activity of acetylsalicylic acid in HCV-expressing cells. Archives of virology. 2014;159:3321-8. [PMID: 25106115] [DOI]
Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, et al. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF‐κB‐inhibiting activity. Cellular microbiology. 2007;9(7):1683-94. [PMID: 17324159] [DOI]
Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265(5174):956-9. [PMID: 8052854] [DOI]
Ma N, Liu X-W, Kong X-J, Li S-H, Jiao Z-H, Qin Z, et al. Aspirin eugenol ester regulates cecal contents metabolomic profile and microbiota in an animal model of hyperlipidemia. BMC Veterinary Research. 2018;14:1-15. [PMID: 30563510] [PMCID: PMC6299661] [DOI]
Wu Y, Wang Y, Yin D, Wu W, Sun X, Zhang Y, et al. Effect of supplementation of nicotinamide and sodium butyrate on the growth performance, liver mitochondrial function and gut microbiota of broilers at high stocking density. Food & Function. 2019;10(11):7081-90. [PMID: 31670358] [DOI]
Guo X, Zhao W, Yin D, Mei Z, Wang F, Tiedje J, et al. Aspirin altered antibiotic resistance genes response to sulfonamide in the gut microbiome of zebrafish. Environmental Pollution. 2024;359:124566. [PMID: 39025292] [DOI]
Lorenzoni A, Rojas-Núñez I, Moore A. Effect of aspirin on the intestinal response to a necrotic enteritis challenge. Avian diseases. 2019;63(4):686-92. [PMID: 31865684] [DOI]
Wang H, Latorre JD, Bansal M, Abraha M, Al-Rubaye B, Tellez-Isaias G, et al. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Scientific reports. 2019;9(1):14541. [PMID: 31601882] [PMCID: PMC6787040] [DOI]
Singh DP, Borse SP, Nivsarkar M. Clinical importance of nonsteroidal anti-inflammatory drug enteropathy: the relevance of tumor necrosis factor as a promising target. Translational Research. 2016;175:76-91. [PMID: 27083387] [DOI]
Wu D, Zhang M, Xu J, Song E, Lv Y, Tang S, et al. In vitro evaluation of aspirin-induced HspB1 against heat stress damage in chicken myocardial cells. Cell Stress and Chaperones. 2016;21(3):405-13. [PMID: 26910344] [PMCID: PMC4837179] [DOI]
Tang S, Zhou S, Yin B, Xu J, Di L, Zhang J, et al. Heat stress-induced renal damage in poultry and the protective effects of HSP60 and HSP47. Cell Stress and Chaperones. 2018;23(5):1033-40. [PMID: 29779133] [PMCID: PMC6111100] [DOI]
Balog JM, Huff GR, Rath NC, Huff WE. Effect of Dietary Aspirin on Ascites in Broilers Raised in a Hypobaric Chamber1. Poultry Science. 2000;79(8):1101-5. [PMID: 10947177] [DOI]
Wu D, Zhang M, Lu Y, Tang S, Kemper N, Hartung J, et al. Aspirin-induced heat stress resistance in chicken myocardial cells can be suppressed by BAPTA-AM in vitro. Cell Stress and Chaperones. 2016;21(5):817-27. [PMID: 27262845] [PMCID: PMC5003798] [DOI]
Salah AS, Mahmoud MA, Ahmed-Farid OA, El-Tarabany MS. Effects of dietary curcumin and acetylsalicylic acid supplements on performance, muscle amino acid and fatty acid profiles, antioxidant biomarkers and blood chemistry of heat-stressed broiler chickens. Journal of thermal biology. 2019;84:259-65. [PMID: 31466762] [DOI]
Sandoghchian Shotorbani MR, Sandooghchian S, Sandoghchian B. Effect of prostaglandins and anti prostaglandins (NSAID) on ascites in broilers. Journal of Biotechnology. 2008;136:S260. [DOI]
SARACILA M, PANAITE TD, VLAICU PA, TABUC C, PALADE ML, GAVRIS T, et al. Dietary Willow Bark Extract for Broilers Reared Under Heat Stress. Bulletin of the University of Agricultural Sciences & Veterinary Medicine Cluj-Napoca Animal Science & Biotechnologies. 2018;75(2). [DOI]
Karam I, Ma N, Liu X-W, Li S-H, Kong X-J, Li J-Y, et al. Regulation effect of Aspirin Eugenol Ester on blood lipids in Wistar rats with hyperlipidemia. BMC veterinary research. 2015;11:1-7. [PMID: 26289078] [PMCID: PMC4546030] [DOI]
Li J, Yu Y, Yang Y, Liu X, Zhang J, Li B, et al. A 15-day oral dose toxicity study of aspirin eugenol ester in Wistar rats. Food and Chemical Toxicology. 2012;50(6):1980-5. [PMID: 22516304] [DOI]
El-Kholy MS, El-Hindawy MM, Alagawany M, Abd El-Hack ME, El-Sayed SAA. Use of acetylsalicylic acid as an allostatic modulator in the diets of growing Japanese quails exposed to heat stress. Journal of Thermal Biology. 2018;74:6-13. [PMID: 29801651] [DOI]
Puron D, Santamaria R, Segura JC. Effects of Sodium Bicarbonate, Acetylsalicylic, and Ascorbic Acid on Broiler Performance in a Tropical Environment. Journal of Applied Poultry Research. 1994;3(2):141-5. [DOI]
Roussan DA, Khwaldeh GY, Haddad RR, Shaheen IA, Salameh G, Al Rifai R. Effect of Ascorbic Acid, Acetylsalicylic Acid, Sodium Bicarbonate, and Potassium Chloride Supplementation in Water on the Performance of Broiler Chickens Exposed to Heat Stress1. Journal of Applied Poultry Research. 2008;17(1):141-4. [DOI]
Published
Submitted
Revised
Accepted
Issue
Section
License
Copyright (c) 2024 Yadollah Badakhshan (Corresponding Author); Arsalan Barazandeh, Morteza Mokhtari (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.