Dopaminergic Receptor Involvement in Insulin-Induced Anorexia in Broiler Chickens
Keywords:
Dopaminergic receptors , Insulin, Feed intake, BroilerAbstract
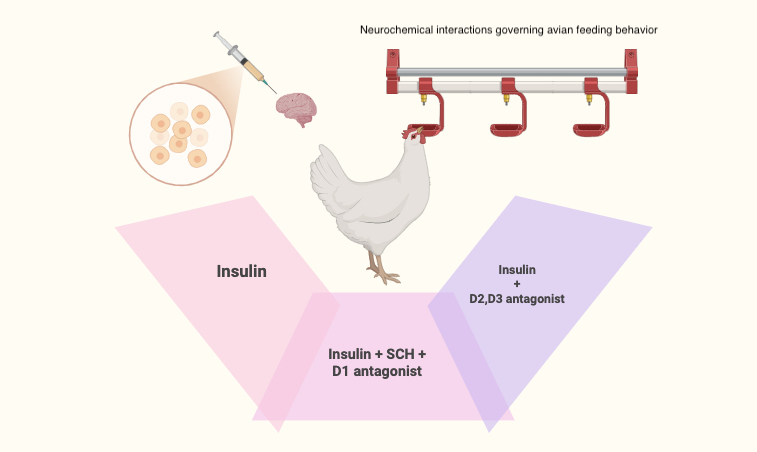
The neurobiological mechanisms underlying appetite regulation and feeding behavior exhibit considerable complexity and interspecies variation. Among the key neurotransmitters implicated in the modulation of feeding behavior are dopamine and insulin, yet the interplay between these signaling molecules remains inadequately characterized. This investigation aimed to elucidate the interactions between insulin and the dopaminergic system in the context of appetite regulation in broiler-type chickens (Ross 308). Experimental protocols involved the intracerebroventricular (ICV) administration of insulin at doses of 2.5, 5, and 10 ng, respectively. Additionally, dopaminergic agents, including L-DOPA (a dopamine precursor) and receptor-specific antagonists SCH 23390 (D1), AMI-193 (D2), NGB 2904 (D3), and L-741,742 (D4), were administered alone or in combination with insulin (10 ng). Meal consumption was quantified cumulatively at 30-, 60-, and 120-minute intervals following the infusion. The findings revealed that insulin elicited a dose-dependent suppression of food intake (p < 0.05). Notably, the anorexigenic effect of insulin was attenuated by SCH 23390 (5 nmol) (p < 0.05), implicating D1 receptor-mediated pathways, whereas antagonists targeting D2, D3, and D4 receptors failed to modulate this response (p > 0.05). These results substantiate the critical role of D1 receptors in mediating insulin-induced anorexia in meat-type chickens, thereby advancing our understanding of the neurochemical interactions governing avian feeding behavior.
Downloads
References
Campos A, Port JD, Acosta A. Integrative hedonic and homeostatic food intake regulation by the central nervous system: insights from neuroimaging. Brain sciences. 2022;12(4):431.[PMID: 35447963] [PMCID: PMC9032173] [DOI]
Yi J, Yuan J, Gilbert E, Siegel P, Cline M. Differential expression of appetite‐regulating genes in avian models of anorexia and obesity. Journal of neuroendocrinology. 2017;29(8):e12510.[PMID: 28727208] [DOI]
Kuenzel WJ, Beck MM, Teruyama R. Neural sites and pathways regulating food intake in birds: a comparative analysis to mammalian systems. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 1999;283(4‐5):348-64[DOI]
Mahdavi K, Zendehdel M, Baghbanzadeh A. Central effects of opioidergic system on food intake in birds and mammals: a review. Veterinary research communications. 2023;47(3):1103-14.[PMID: 37209184] [DOI]
Mahdavi K, Zendehdel M, Zarei H. The role of central neurotransmitters in appetite regulation of broilers and layers: similarities and differences. Veterinary Research Communications. 2024;48(3):1313-28.[PMID: 38286893] [DOI]
Saltiel AR. Insulin signaling in the control of glucose and lipid homeostasis. Metabolic control. 2016:51-71.[PMID: 26721672] [DOI]
Marshall S. Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Science's STKE. 2006;2006(346):re7-re.[PMID: 16885148] [DOI]
Blevins JE, Schwartz MW, Baskin DG. Peptide signals regulating food intake and energy homeostasis. Canadian Journal of Physiology and Pharmacology. 2002;80(5):396-406[PMCID: 12056545] [DOI]
Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, et al. The catabolic action of insulin in the brain is mediated by melanocortins. Journal of Neuroscience. 2002;22(20):9048-52.[PMID: 12388611] [PMCID: PMC6757684] [DOI]
Shiraishi J-i, Yanagita K, Fujita M, Bungo T. Central insulin suppresses feeding behavior via melanocortins in chicks. Domestic Animal Endocrinology. 2008;34(3):223-8.[PMID: 17629654] [DOI]
Beaulieu JM, Espinoza S, Gainetdinov RR. Dopamine receptors–IUPHAR R eview 13. British journal of pharmacology. 2015;172(1):1-23.[PMID: 25671228] [PMCID: PMC4280963] [DOI]
Bungo T, Yanagita K, Shiraishi J. Feed intake after infusion of Noradrenalin, Dopamine or its. J Anim Vet Adv. 2010;9:760-3[DOI]
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H. Interaction between opioidergic and dopaminergic systems on food intake in neonatal layer type chicken. International Journal of Peptide Research and Therapeutics. 2016;22:83-92[DOI]
Meyers AM, Mourra D, Beeler JA. High fructose corn syrup induces metabolic dysregulation and altered dopamine signaling in the absence of obesity. PloS one. 2017;12(12):e0190206.[PMID: 29287121] [PMCID: PMC5747444] [DOI]
Baik J-H. Dopaminergic control of the feeding circuit. Endocrinology and Metabolism. 2021;36(2):229-39.[PMID: 33820393] [PMCID: PMC8090468] [DOI]
Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M, et al. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain research. 1991;557(1-2):154-61.[PMID: 1747750] [DOI]
Li L, Li X, Zhou W, Messina JL. Acute psychological stress results in the rapid development of insulin resistance. The Journal of endocrinology. 2013;217(2):175.[PMID: 23444388] [PMCID: PMC3804337] [DOI]
Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nature communications. 2015;6(1):8543.[PMID: 26503322] [PMCID: PMC4624275] [DOI]
Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain research. 1994;644(2):331-4.[PMID: 8050044] [DOI]
Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proceedings of the National Academy of Sciences. 2015;112(11):3463-8.[PMID: 25733901] [PMCID: PMC4371978] [DOI]
Könner AC, Hess S, Tovar S, Mesaros A, Sánchez-Lasheras C, Evers N, et al. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell metabolism. 2011;13(6):720-8.[PMID: 21641553] [DOI]
Yousefvand S, Hamidi F, Zendehdel M, Parham A. Hypophagic effects of insulin are mediated via NPY1/NPY2 receptors in broiler cockerels. Canadian Journal of Physiology and Pharmacology. 2018;96(12):1301-7.[PMID: 30326197] [DOI]
Zendehdel M, Hasani K, Babapour V, Mortezaei SS, Khoshbakht Y, Hassanpour S. Dopamine-induced hypophagia is mediated by D1 and 5HT-2c receptors in chicken. Veterinary research communications. 2014;38:11-9.[PMID: 24122738] [DOI]
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A. Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiology & Behavior. 1979;22(4):693-5.[PMID: 482410] [DOI]
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S. The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. European journal of pharmacology. 1997;339(2-3):211-3.[PMID: 9473137] [DOI]
Saito E-S, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, et al. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regulatory peptides. 2005;125(1-3):201-8.[PMID: 15582733] [DOI]
Shiraishi J-i, Yanagita K, Fukumori R, Sugino T, Fujita M, Kawakami S-I, et al. Comparisons of insulin related parameters in commercial-type chicks: evidence for insulin resistance in broiler chicks. Physiology & behavior. 2011;103(2):233-9.[PMID: 21316379] [DOI]
Dupont J, Tesseraud S, Simon J. Insulin signaling in chicken liver and muscle. General and Comparative Endocrinology. 2009;163(1-2):52-7.[PMID: 18996126] [DOI]
Dupont J, Tesseraud S, Derouet M, Collin A, Rideau N, Crochet S, et al. Insulin immuno-neutralization in chicken: effects on insulin signaling and gene expression in liver and muscle. Journal of endocrinology. 2008;197(3):531-42.[PMID: 18492818] [DOI]
Small L, Brandon AE, Turner N, Cooney GJ. Modeling insulin resistance in rodents by alterations in diet: what have high-fat and high-calorie diets revealed? American Journal of Physiology-Endocrinology and Metabolism. 2018;314(3):E251-E65.[PMID: 29118016] [DOI]
Rojas J, Printz R, Niswender K. Insulin detemir attenuates food intake, body weight gain and fat mass gain in diet-induced obese Sprague–Dawley rats. Nutrition & diabetes. 2011;1(7):e10-e.[PMID: 23449422] [PMCID: PMC3302138] [DOI]
Terry P, Katz JL. Differential antagonism of the effects of dopamine D 1-receptor agonists on feeding behavior in the rat. Psychopharmacology. 1992;109:403-9.[PMID: 1365854] [DOI]
Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497-506.[PMID: 17031710] [DOI]
Li Y, South T, Han M, Chen J, Wang R, Huang X-F. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain research. 2009;1268:181-9.[PMID: 19285041] [DOI]
Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, et al. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proceedings of the National Academy of Sciences. 2016;113(9):2520-5.[PMID: 26884159] [PMCID: PMC4780604] [DOI]
Liu S, Labouèbe G, Karunakaran S, Clee S, Borgland S. Effect of insulin on excitatory synaptic transmission onto dopamine neurons of the ventral tegmental area in a mouse model of hyperinsulinemia. Nutrition & diabetes. 2013;3(12):e97-e.[PMID: 24336291] [PMCID: PMC3877429] [DOI]
Downloads
Published
Submitted
Revised
Accepted
Issue
Section
License
Copyright (c) 2024 Hamed Zarei (Corresponding Author); Shayan Biglari, Morteza Zendehdel (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.