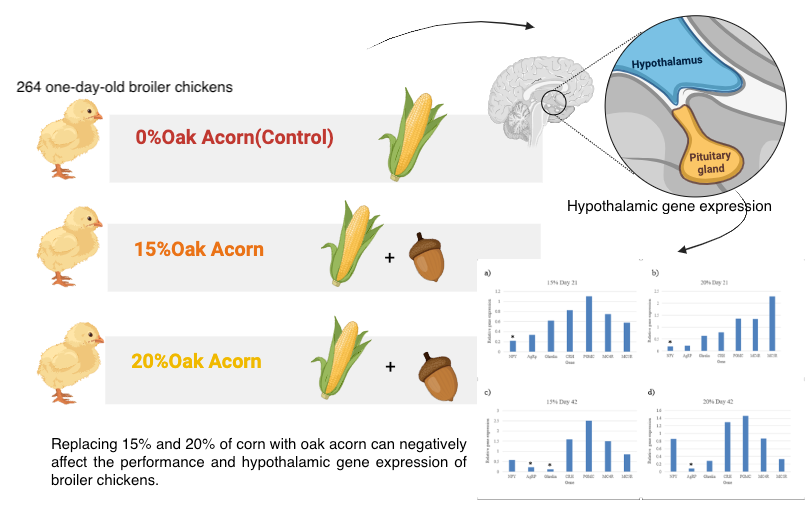
Effects of Oak Acorn on Performance and Gene Expression of Hypothalamus Tissue in Broiler Chickens
Keywords:
Gene expression, Hypothalamus, Oak acorn, BroilerAbstract
Oak fruit is high in energy and, therefore, can be used as a substitute for corn in poultry diets. However, one limitation of oak fruit is its high level of antinutritional compounds (tannins). This study investigated the effects of corn replacement with oak acorns on performance traits and the mRNA levels of hypothalamic genes in broiler chickens. For this purpose, a total of 264 one-day-old broiler chickens were randomly assigned to three experimental treatments (0, 15, and 20 % oak acorn). Body weight gain, feed intake, and feed conversion were calculated on a pen basis at 21 and 42 days of age. The results showed that body weight gain and feed conversion were significantly affected by treatments at both 21 and 42 days of age; however, no significant difference was observed in feed intake among the treatments. At the age of 21 days, a significant difference in weight gain was observed among the three treatments, whereas this difference was not significant for the treatments with oak acorn on day 42. However, the lowest weight gain was observed in the 20% oak acorn diet at both ages. Feed conversion was significantly higher in 20% of the oak acorn treatment relative to the control group on day 42, while feed conversion was not affected by treatments at the age of 21 days. In addition, the mRNA levels of NPY, AgRP, and Ghrelin were significantly downregulated in the hypothalamus tissue of broilers fed diets containing oak acorn. At the age of 21 days, the expression levels of NPY showed a significant decrease in the hypothalamus tissue of broilers fed with 15% and 20% oak corn diets, while this decrease was significant for the AgRp and Ghrelin genes on day 42. In conclusion, these results suggest that replacing 15% and 20% of corn with oak acorn can negatively affect the performance and hypothalamic gene expression of broiler chickens.
Downloads
References
Saeidi F, Houshmand M, Parsaei S, Zarrin M. Potential of oak acorn with and without polyethylene glycol as an alternative to corn in broiler diets. South African Journal of Animal Science. 2017;47(6):895-903. [DOI]
Bouderoua K, Mourot J, Selselet-Attou G. The effect of green oak acorn (Quercus ilex) based diet on growth performance and meat fatty acid composition of broilers. Asian-Australasian journal of animal sciences. 2009;22(6):843-8. [DOI]
Cicogana M CL, Pialorsi G, Gardella, D, D. LC. . Experiments on the possibility of replacing maize with rice germ acorn and suerosain mixtures for meat chickens. Rivstadi, Zootecnia, . 1973;45:187-200.
Lamy E, Pinheiro C, Rodrigues L, Capela-Silva F, Lopes O, Tavares S, et al. Determinants of tannin-rich food and beverage consumption: oral perception vs. psychosocial aspects. 2016.
Butler LG. Effects of condensed tannin on animal nutrition. Chemistry and significance of condensed tannins: Springer; 1989. p. 391-402. https://doi.org/10.1007/978-1-4684-7511-1_24} 3_https://doi.org/DOI}
Jansman A. Tannins in feedstuffs for simple-stomached animals. Nutrition research reviews. 1993;6(1):209-36. [PMID: 19094309] [DOI]
Mahmood S, Khan AM, Sarwar M, Nisa M, Lee W, Kim S, et al. Use of chemical treatments to reduce tannins and trypsin inhibitor contents in salseed (Shorea robusta) meal. Asian-australasian journal of animal sciences. 2007;20(9):1462-7. [DOI]
Mahmood S, Khan MA, Sarwar M, Nisa M. Use of chemical treatments to reduce antinutritional effects of tannins in salseed meal: Effect on performance and digestive enzymes of broilers. Livestock Science. 2008;116(1-3):162-70. [DOI]
Oduhu G, Baker D. Some tropical high tannin sorghums and their effects on broiler performance. Agricultura Tropica et Subtropica. 2005;38:105-10.
Ortiz L, Alzueta C, Trevino J, Castano M. Effects of faba bean tannins on the growth and histological structure of the intestinal tract and liver of chicks and rats. British Poultry Science. 1994;35(5):743-54. [PMID: 7719738] [DOI]
Robbins CT, Mole S, Hagerman A, Hanley T. Role of tannins in defending plants against ruminants: reduction in dry matter digestion? Ecology. 1987;68(6):1606-15. [PMID: 29357186] [DOI]
Silanikove N, Gilboa N, Perevolotsky A, Nitsan Z. Goats fed tannin-containing leaves do not exhibit toxic syndromes. Small Ruminant Research. 1996;21(3):195-201. [DOI]
Hirscbberg AL. Hormonal regulation of appetite and food intake. Annals of medicine. 1998;30(1):7-20. [PMID: 9556085] [DOI]
Bloom S. Hormonal regulation of appetite. obesity reviews. 2007;8:63-5. [PMID: 17316304] [DOI]
Takeda E, Terao J, Nakaya Y, Miyamoto K-i, Baba Y, Chuman H, et al. Stress control and human nutrition. The Journal of Medical Investigation. 2004;51(3, 4):139-45. [PMID: 15460899] [DOI]
Kuenzel WJ, Masson M. A Stereotaxic Atlas of the Brain of the Chick (Gallus domesticus): Johns Hopkins University Press; 1988. 3_https://doi.org/DOI}
Panickar KS. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Molecular nutrition & food research. 2013;57(1):34-47. [PMID: 23125162] [DOI]
Furuse M, Tachibana T, Ohgushi A, Ando R, Yoshimatsu T, Denbow DM. Intracerebroventricular injection of ghrelin and growth hormone releasing factor inhibits food intake in neonatal chicks. Neuroscience letters. 2001;301(2):123-6. [PMID: 11248438] [DOI]
Kim SO, Yun S-J, Lee EH. The water extract of adlay seed (Coix lachrymajobi var. mayuen) exhibits anti-obesity effects through neuroendocrine modulation. The American journal of Chinese medicine. 2007;35(02):297-308. [PMID: 17436369] [DOI]
Ahmed A, Smithard R, Ellis M. Activities of enzymes of the pancreas, and the lumen and mucosa of the small intestine in growing broiler cockerels fed on tannin-containing diets. British Journal of Nutrition. 1991;65(2):189-97. [PMID: 2043603] [DOI]
Al-Mamary M, Molham A-H, Abdulwali A-A, Al-Obeidi A. In vivo effects of dietary sorghum tannins on rabbit digestive enzymes and mineral absorption. Nutrition Research. 2001;21(10):1393-401. [DOI]
Mansoori B, Acamovic T. The effect of tannic acid on the excretion of endogenous methionine, histidine and lysine with broilers. Animal Feed Science and Technology. 2007;134(3-4):198-210. [DOI]
Te Pas MF, Borg R, Buddiger NJ, Wood BJ, Rebel JM, van Krimpen MM, et al. Regulating appetite in broilers for improving body and muscle development–A review. Journal of Animal Physiology and Animal Nutrition. 2020;104(6):1819-34. [PMID: 32592266] [PMCID: PMC7754290] [DOI]
Argente-Arizón P, Freire-Regatillo A, Argente J, Chowen JA. Role of non-neuronal cells in body weight and appetite control. Frontiers in endocrinology. 2015;6:42. [PMID: 25859240] [PMCID: PMC4374626] [DOI]
Myoung H-J, Kim G, Nam K-W. Apigenin isolated from the seeds of Perilla frutescens britton var crispa (Benth.) inhibits food intake in C57BL/6J mice. Archives of pharmacal research. 2010;33(11):1741-6. [PMID: 21116776] [DOI]
Sternson SM, Atasoy D. Agouti-related protein neuron circuits that regulate appetite. Neuroendocrinology. 2014;100(2-3):95-102. [PMID: 25402352] [DOI]
Olszewski PK, Bomberg EM, Grace MK, Levine AS. α-Melanocyte stimulating hormone and ghrelin: Central interaction in feeding control. Peptides. 2007;28(10):2084-9. [PMID: 17719137] [DOI]
Boswell T, Takeuchi S. Recent developments in our understanding of the avian melanocortin system: its involvement in the regulation of pigmentation and energy homeostasis. Peptides. 2005;26(10):1733-43. [PMID: 15978703] [DOI]
Gantz I, Fong TM. The melanocortin system. American Journal of Physiology-Endocrinology and Metabolism. 2003;284(3):E468-E74. [PMID: 12556347] [DOI]
Saito E-s, Kaiya H, Takagi T, Yamasaki I, Denbow DM, Kangawa K, et al. Chicken ghrelin and growth hormone-releasing peptide-2 inhibit food intake of neonatal chicks. European journal of pharmacology. 2002;453(1):75-9. [PMID: 12393062] [DOI]
Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surgical neurology international. 2010;1. [PMID: 21206541] [PMCID: PMC3011108] [DOI]
Lagha AB, Grenier D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Scientific Reports. 2016;6(1):1-11. [PMID: 27694921] [PMCID: PMC5046134] [DOI]
Kishimoto M, Okimura Y, Nakata H, Kudo T, Iguchi G, Takahashi Y, et al. Cloning and characterization of the 5′-flanking region of the human ghrelin gene. Biochemical and Biophysical Research Communications. 2003;305(1):186-92. [PMID: 12732215] [DOI]
Halter BA. Effects of exogenous and endogenous factors on appetite regulation in broiler chicks and Japanese quail: Virginia Tech; 2021.
Downloads
Published
Submitted
Revised
Accepted
Issue
Section
License
Copyright (c) 2024 Jafar Studeh (Author); Mustafa Muhaghegh Dolatabady (Corresponding Author); Asma Moradalipour, Mohammad Hooshmand (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.