Immunogenetics Properties of Avian MHC Polymorphism and its Association with Diseases and Production Traits
Keywords:
Allelic polymorphism, MHC, Avian, Immunogenetics, Resistance, Production traitsAbstract
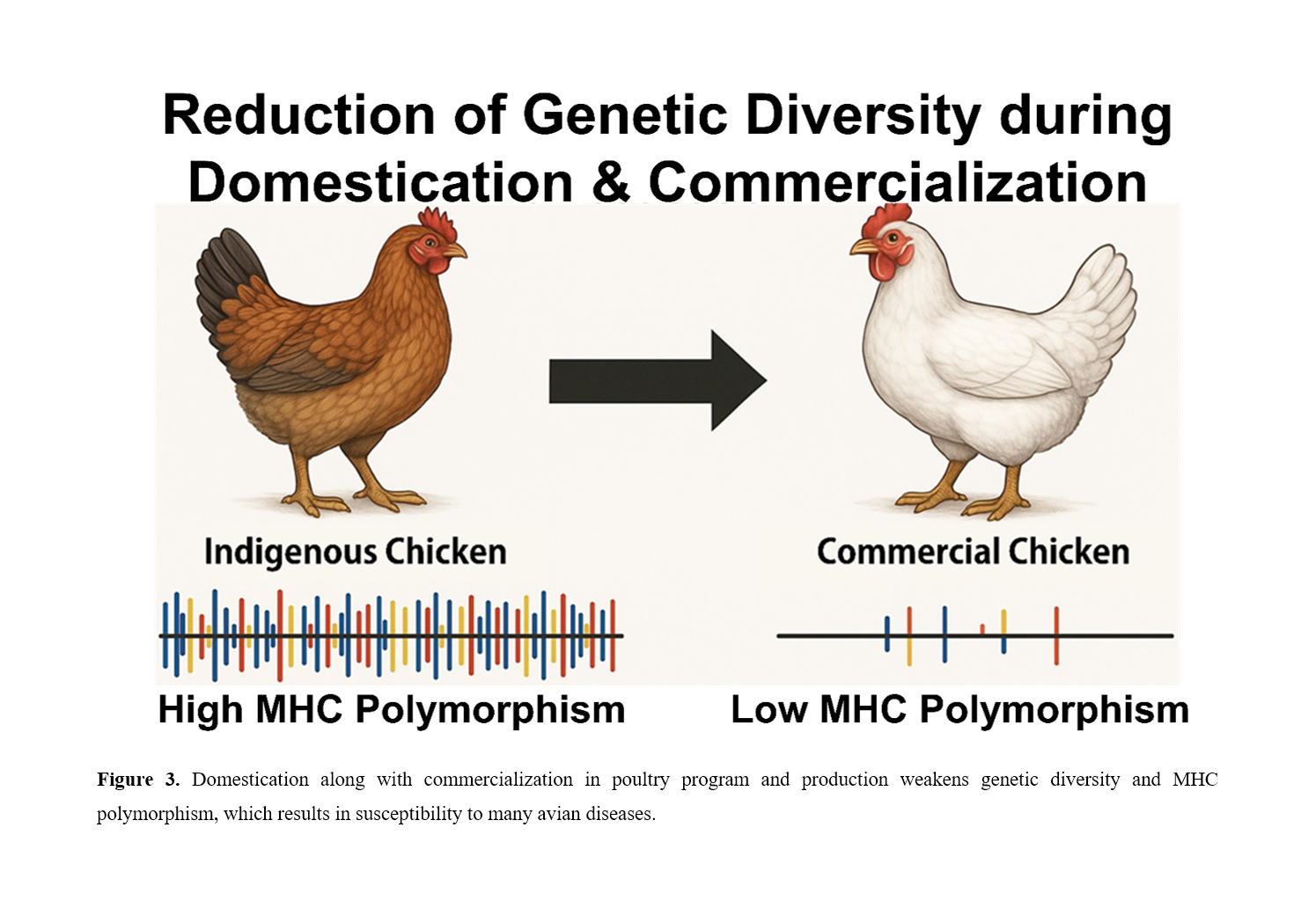
Avian immunogenetics has emerged as a critical field in understanding the genetic basis of disease resistance and immune competence in poultry. Though structurally simpler than its mammalian counterpart, the Major Histocompatibility Complex (MHC) in chickens plays a pivotal role in pathogen defense due mainly to MHC’s high polymorphism and dominant allelic expression patterns. Unlike mammals, where MHC class I and II molecules exhibit co-dominant expression, chickens predominantly express a single MHC class I molecule, leading to a binary resistance/susceptibility outcome against specific pathogens; their polymorphisms influence not only immunity but also economically valuable traits (i.e., growth and egg production). This unique feature has facilitated extensive research linking MHC alleles to disease outcomes, including resistance to infectious bursal disease virus (IBDV), Marek’s disease (MD), and avian influenza. Beyond immunity, MHC polymorphisms correlate with economically significant traits such as egg production and growth rates, underscoring their dual role in health and production. However, modern poultry breeding programs often overlook genetic diversity, prioritizing production traits at the expense of immunocompetence. Integrating MHC-based marker-assisted breeding into poultry programs is essential to preserve genetic diversity and enhance immunocompetence. By leveraging advances in genomics and immunogenetics, future research can optimize poultry health, ensuring sustainable production in the face of evolving pathogen threats. This review highlights the importance of MHC polymorphism for both disease management and poultry economics; particularly, the study’s strength lies in its presentation of allele-specific immunocompetence patterns across viral, bacterial, and parasitic pathogens, easing integrating MHC-based marker-assisted selection into breeding strategies to enhance disease resistance while maintaining genetic variability. These insights advance avian immunology and offer practical applications for improving global poultry welfare and food security.
Downloads
References
Almajwal A, Alam I, Zeb F, Fatima S. Energy Metabolism and Allocation in Selfish Immune System and Brain: A Beneficial Role of Insulin Resistance in Aging. Food Nutr Sci. 2019;10:64-80.
Nonić M, Šijačić-Nikolić M. Genetic Diversity: Sources, Threats, and Conservation. Springer Cham2019. p. 1-15.
Burgess SC. Proteomics in the chicken: Tools for understanding immune responses to avian diseases. Poultry Science. 2004:552-73.
Lenardo M, Lo B, Lucas CL. Genomics of Immune Diseases and New Therapies. Annual Review of Immunology. 2016;34:121-49.
Chaves LD, Krueth SB, Reed KM. Defining the Turkey MHC: Sequence and Genes of the B Locus. J Immunol. 2009;183:6530-7.
Ekblom R, Stapley J, Ball AD, Birkhead T, Burke T, Slate J. Genetic mapping of the major histocompatibility complex in the zebra finch (Taeniopygia guttata). Immunogenetics. 2011;63:523-30.
McDermid EM. Immune-genetics of the Chicken. Vox Sanguinis. 1964:249-67.
Moon DA, Veniamin SM, Parks-Dely JA, Magor KE. The MHC of the Duck (Anas platyrhynchos) Contains Five Differentially Expressed Class I Genes. J Immunol. 2005;175:6702-12.
Magor KE, Miranzo Navarro D, Barber MRW, Petkau K, Fleming-Canepa X, Blyth G. Defense genes missing from the flight division. Dev Comp Immunol. 2013;41:377-88.
Brujeni GN, Houshmand P, Esmailnejad A, Abasabadi F. Major Histocompatibility Complex as Marker Assisted Selection for Breeding Immunocompetent Animal. Iranian Journal of Veterinary Medicine. 2022;16:211-27.
Briles WE, Briles RW. Genetics and classification of major histocompatibility complex antigens of the chicken. Poult Sci. 1987;66:776-81.
Elwood Briles W, Briles RW, Taffs RE, Stone HA. Resistance to a malignant lymphoma in chickens is mapped to subregion of major histocompatibility (B) complex. Science. 1983;219:977-9.
Kaufman J. Innate immune genes of the chicken MHC and related regions. Immunogenetics. 2022;74:167-77.
Fillon V, Zoorob R, Yerle M, Auffray C, Vignal A. Mapping of the genetically independent chicken major histocompatibility complexes B@ and RFP-Y@ to the same microchromosome by two-color fluorescent in situ hybridization. Cytogenet Cell Genet. 1996;75:7-9.
Guillemot F. The chicken major histocompatibility complex (MHC): Evolutionary conserved class I and class II genes are closely associated with non-MHC genes. Integr Comp Biol. 1991;31:592-7.
Guillemot F, Billault A, Pourquié O, Béhar G, Chaussé AM, Zoorob R, et al. A molecular map of the chicken major histocompatibility complex: the class II beta genes are closely linked to the class I genes and the nucleolar organizer. EMBO J. 1988;7:2775-85.
Yuan Y, Zhang H, Yi G, You Z, Zhao C, Yuan H. Genetic Diversity of MHC B-F/B-L Region in 21 Chicken Populations. Front Genet. 2021;12:1-10.
Trowsdale J, Knight JC. Major histocompatibility complex genomics and human disease. Annual Review of Genomics and Human Genetics. 2024;14:301-23.
da Silva AP, Gallardo RA. The chicken MHC: Insights into genetic resistance, immunity, and inflammation following infectious bronchitis virus infections. Vaccines. 2020:1-15.
Fulton JE. Advances in methodologies for detecting MHC-B variability in chickens. Poultry Science. 2020;99:1267-74.
Miller MM, Taylor RL. Brief review of the chicken Major Histocompatibility Complex: The genes, their distribution on chromosome 16, and their contributions to disease resistance. Poult Sci. 2016;95:375-92.
Lamont SJ. The chicken major histocompatibility complex and disease. OIE Rev Sci Tech. 1998;17:128-42.
Kaufman J. Generalists and Specialists: A New View of How MHC Class I Molecules Fight Infectious Pathogens. Trends in Immunology. 2018;39:367-79.
Walker BA, Hunt LG, Sowa AK, Skjødt K, Göbel TW, Lehner PJ. The dominantly expressed class I molecule of the chicken MHC is explained by coevolution with the polymorphic peptide transporter (TAP) genes. Proc Natl Acad Sci USA. 2011;108:8396-401.
Fadly AM, Bacon LD. Response of B congenic chickens to infection with infectious bursal disease virus. Avian Dis. 1992;36:871-80.
Hepkema BG, Hensen EJ, Blankert JJ, van der Zijpp AJ, Albers GAA, Tilanus MGJ. Mapping of susceptibility to Marek's disease within the major histocompatibility (B) complex by refined typing of White Leghorn chickens. Anim Genet. 1993;24:283-7.
Banat GR, Tkalcic S, Dzielawa JA, Jackwood MW, Saggese MD, Yates L. Association of the chicken MHC B haplotypes with resistance to avian coronavirus. Dev Comp Immunol. 2013;39:430-7.
Owen JP, Delany ME, Mullens BA. MHC haplotype involvement in avian resistance to an ectoparasite. Immunogenetics. 2008;60:621-31.
Schou T, Permin A, Roepstorff A, Sørensen P, Kjær J. Comparative genetic resistance to Ascaridia galli infections of 4 different commercial layer-lines. Br Poult Sci. 2003;44:182-5.
Joiner KS, Hoerr FJ, Van Santen E, Ewald SJ. The avian major histocompatibility complex influences bacterial skeletal disease in broiler breeder chickens. Vet Pathol. 2005;42:275-81.
Cotter PF, Taylor RL, Abplanalp H. B-Complex Associated Immunity to Salmonella enteritidis Challenge in Congenic Chickens. Poult Sci. 1998;77:1846-51.
Collins WM, Briles WE, Zsigray RM, Dunlop WR, Corbett AC, Clark KK. The B locus (MHC) in the chicken: Association with the fate of RSV-induced tumors. Immunogenetics. 1977;5:333-43.
Lwelamira J, Kifaro GC, Gwakisa PS. Genetic parameters for body weights, egg traits and antibody response against Newcastle Disease Virus (NDV) vaccine among two Tanzania chicken ecotypes. Trop Anim Health Prod. 2009;41:51-9.
Hunt HD, Jadhao S, Swayne DE. Major histocompatibility complex and background genes in chickens influence susceptibility to high pathogenicity avian influenza virus. Avian Diseases. 2010:572-5.
Null BH, Sheldon BL. Association of the major histocompatibility complex with avian leukosis virus infection in chickens. Br Poult Sci. 1992;33:613-20.
Macklin KS, Ewald SJ, Norton RA. Major histocompatibility complex effect on cellulitis among different chicken lines. Avian Pathol. 2002;31:371-6.
Poulsen DJ, Thureen DR, Keeler CL. Comparison of Disease Susceptibility and Resistance in Three Lines of Chickens Experimentally Infected with Infectious Laryngotracheitis Virus. Poult Sci. 1998;77:17-21.
Nikbakht G, Esmailnejad A. Chicken major histocompatibility complex polymorphism and its association with production traits. Immunogenetics. 2015;67:247-52.
Haunshi S, Devara D, Ramasamy K, Ullengala R, Nath Chatterjee R. Genetic diversity at major histocompatibility complex and its effect on production and immune traits in indigenous chicken breeds of India. Arch Anim Breed. 2020;63:173-82.
Bacon LD. Influence of the major histocompatibility complex on disease resistance and productivity. Poult Sci. 1987;66:802-11.
Esmailnejad A, Nikbakht Brujeni GR. Study of MHC polymorphism and its linkage to IGF1 gene in Khorasan indigenous chicken. J Vet Res. 2016;71:481-789.
Habimana R, Ngeno K, Okeno TO, Hirwa CA, Tiambo CK, Yao NK. Genome-Wide Association Study of Growth Performance and Immune Response to Newcastle Disease Virus of Indigenous Chicken in Rwanda. Front Genet. 2021;12:1-13.
Kaiser M, Kaufman J, Lamont SJ. Different MHC class I cell surface expression levels in diverse chicken lines, associations with B blood group, and proposed relationship to antigen-binding repertoire. Poult Sci. 2025;104:1-10.
Kim CD, Lamont SJ, Rothschild MF. Associations of major histocompatibility complex haplotypes with body weight and egg production traits in S1 White Leghorn chickens. Poult Sci. 1989;68:464-9.
Kim M, Ediriweera TH, Cho E, Chung Y, Manjula P, Yu M, et al. Major histocompatibility complex genes exhibit a potential immunological role in mixed Eimeria-infected broiler cecum analyzed using RNA sequencing. Anim Biosci. 2024;37:993-1000.
Nikbakht G, Esmailnejad A, Barjesteh N. LEI0258 microsatellite variability in Khorasan, Marandi, and Arian chickens. Biochem Genet. 2013;51:341-9.
Pan R, Qi L, Xu Z, Zhang D, Nie Q, Zhang X, et al. Weighted single-step GWAS identified candidate genes associated with carcass traits in a Chinese yellow-feathered chicken population. Poult Sci. 2024;103.
Xu W, Wang Z, Qu Y, Li Q, Tian Y, Chen L, et al. Genome-Wide Association Studies and Haplotype-Sharing Analysis Targeting the Egg Production Traits in Shaoxing Duck. Front Genet. 2022;13.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Jalil Mehrzad (Corresponding Author); Pouya Houshmand (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.